A fluid is in “supercritical state” when it is placed at a temperature above its critical temperature and at a pressure above its critical pressure. Above these conditions there is no frontier between liquid and gas, and small changes in the temperature or pressure could result in continuous changes in the fluid’s density, solvent power and other physical properties.
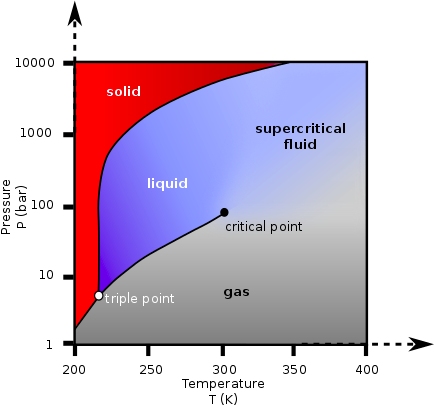
Supercritical Fluids can be placed in conditions where they have several advantageous properties of both liquids and gases, leading for instance to much faster mass transfer rates which is very interesting for extraction or reaction, or high diffusivity and low viscosity with a lot of application on highly porous materials processing, extraction impregnation, nano-particles formation etc….
| Density (kg/m3) | Viscosity (µPa.sec) | Diffusivity (mm2/s) | |
|---|---|---|---|
| Gases | 1 | 10 | 1–10 |
| Supercritical fluids | 100–1000 | 50–100 | 0.01–0.1 |
| Liquids | 1000 | 500–1000 | 0.001 |
Comparison of gases, supercritical fluids and liquids properties
The two most widely used Supercritical Fluids are carbon dioxide (CO2) and water (H2O). Both fluids are readily available and can be used in a pressurized formed to replace organic solvents or being alternative environment friendly processes.
| Solvent | Critical temperature (°C ) | Critical pressure (Bar ) | Critical density (g/cm3 ) |
|---|---|---|---|
| Carbon dioxide (CO2) | 31.1 | 73,8 | 0.469 |
| Water (H2O) | 374 | 220 | 0.322 |
| Propane (C3H8) | 96,7 | 42,5 | 0.162 |
More on supercritical fluid on Wikipedia
Contact us to know more about supercritical fluid potential for your product